Kenya's Pharmacy and Poisons Board has confirmed that it has approved for emergency use in Kenya Russia's Covid-19 vaccine, Sputnik V, following locally done tests.
The board said it authorised the use of Sputnik V jab after a successful evaluation process.
“This is not a registration. The application for Emergency Use Authorization of Russia’s Sputnik V vaccine has been approved after it met all requirements,” said the board.
In a statement issued on Wednesday, the Pharmacy and Poisons Board revealed that it had looked into all aspects of quality, safety and efficacy in their reviewing of the Russian jab.
“It is wholesomely safe. The Board continues to review the safety of all authorized products in the market,” it added.
Read More
The board’s mandate is to authorise and safely monitor medicines and health technologies applied in the country to ensure their safety to Kenyans.
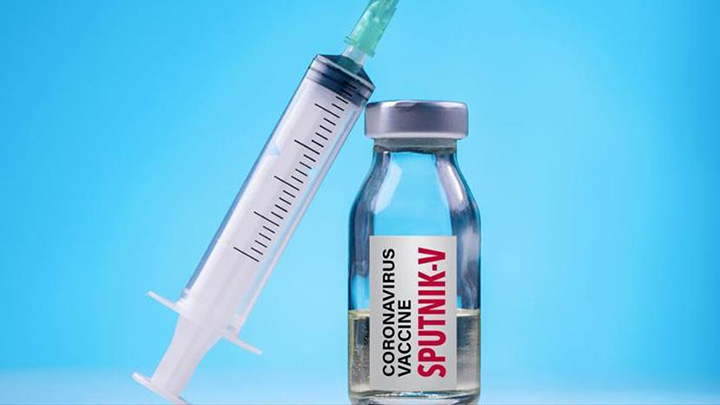
Sputnik V vaccine. PHOTO/COURTESY
But the Health Ministry has the duty to choose which vaccine to buy based on cost and cold chain considerations, while observing National Vaccine and Immunization Programme guidelines.
The board already approved AstraZeneca jab for use in Kenya and is in the process of evaluating Pfizer and Sinovac vaccines before giving authorization for emergency use.
Covid-19 vaccines essentially prepare the body to be able to fight serious effects of the SARS-CoV-2 virus and reduce chances of death in those who have contracted the virus.
Sputnik V is developed by Gamaleya National Centre of Epidemiology and Microbiology.








-1757243598.jpg)
-1757244564.jpg)
