The Common Market for Eastern and Southern Africa (COMESA) Competition Commission has alerted its member states to the circulation of a counterfeit cancer drug in Kenya, raising serious health concerns across the region.
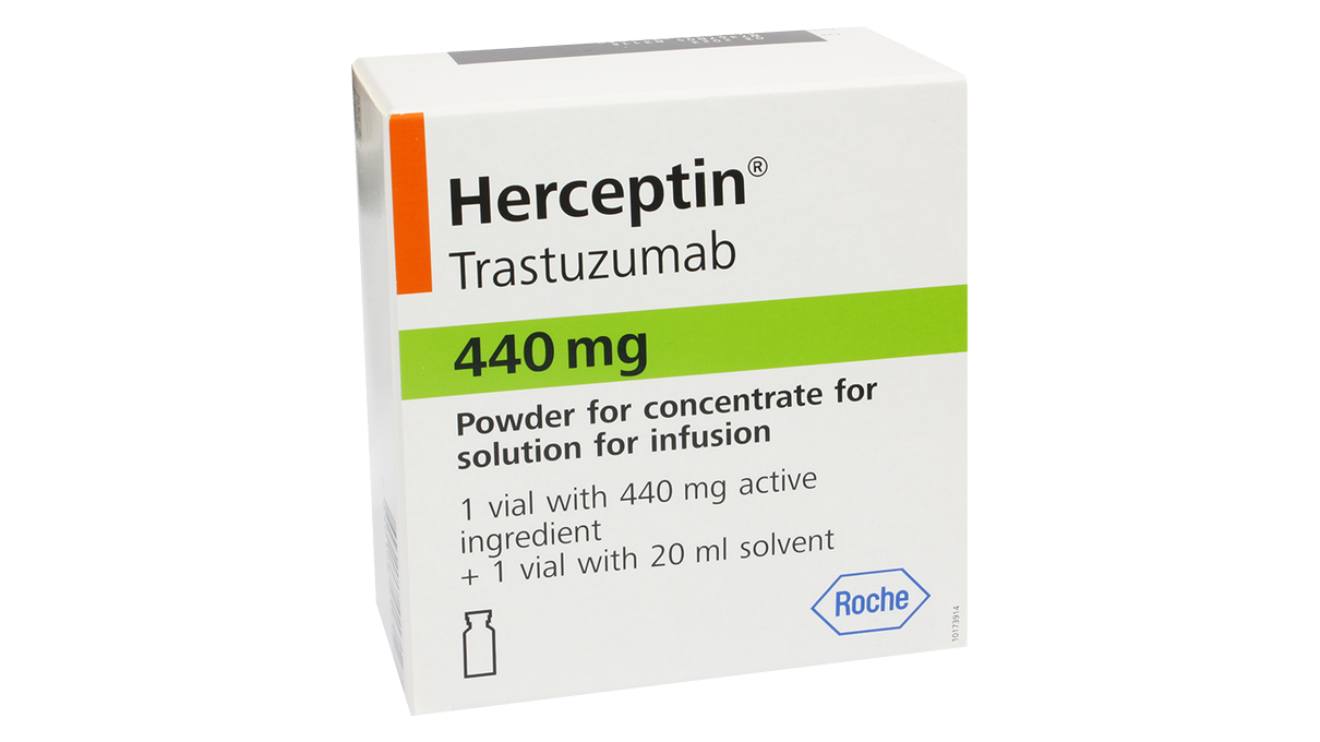
The fake batch of Herceptin 440 mg (Trastuzumab 440 mg) was recently identified in the Kenyan market, prompting an urgent warning from health authorities.
This announcement follows a warning issued by Kenya's Pharmacy and Poisons Board (PPB), which identified the counterfeit drug as a significant threat.
According to the PPB, the falsified Herceptin batch is dangerous, as it lacks proper authorisation and has been confirmed as fake by the brand owner.
In a public notice, the COMESA Competition Commission stated, "The Commission has become aware through the public notice dated May 11 2024 from the Pharmacies and Poisons Board (PPB) of Kenya about a suspicious batch of falsified Herceptin 440 mg (Trastuzumab 440 mg) product that has been detected in the market."
Read More
The Commission fears that this dangerous product might also be distributed to other member states.
The Commission has urged consumers in all member states to be vigilant and avoid purchasing or using this counterfeit drug.
“In this regard, pursuant to Article 30(1)(b) of the COMESA Competition Regulations, the Commission requests any person who establishes that the recalled product is being sold in other COMESA Member States, to avoid its purchase or use, and to report the matter to the Commission,” the statement read.
Fred Siyoi, the CEO of PPB, detailed the specifics of the counterfeit product, which falsely claims to be manufactured by Roshe Products Limited in Germany.
The suspect batch bears the number C5830083, with a manufacturing date of December 2021 and an expiry date of November 2024.
Siyoi confirmed, “It is not authorized to be in the market and is a falsified product in view of the falsified contents, packaging and labeling aspects which have been confirmed by the brand owner.”
The PPB first discovered the counterfeit medication circulating within Kenya, highlighting its severe health risks.
Kenya’s health authorities have assured the public that they maintain rigorous surveillance and quality control systems to monitor the safety of medical products in the market.
As this counterfeit drug poses a substantial risk to patient safety, the COMESA Commission and PPB's swift action aim to prevent further distribution and protect public health across the region.

-1731022418.jpg)
-1730442410.jpg)
 (1)-1730070935.jpg)
 (1)-1727972556.jpg)

-1731675695.jpg)

-1731665904.jpg)
