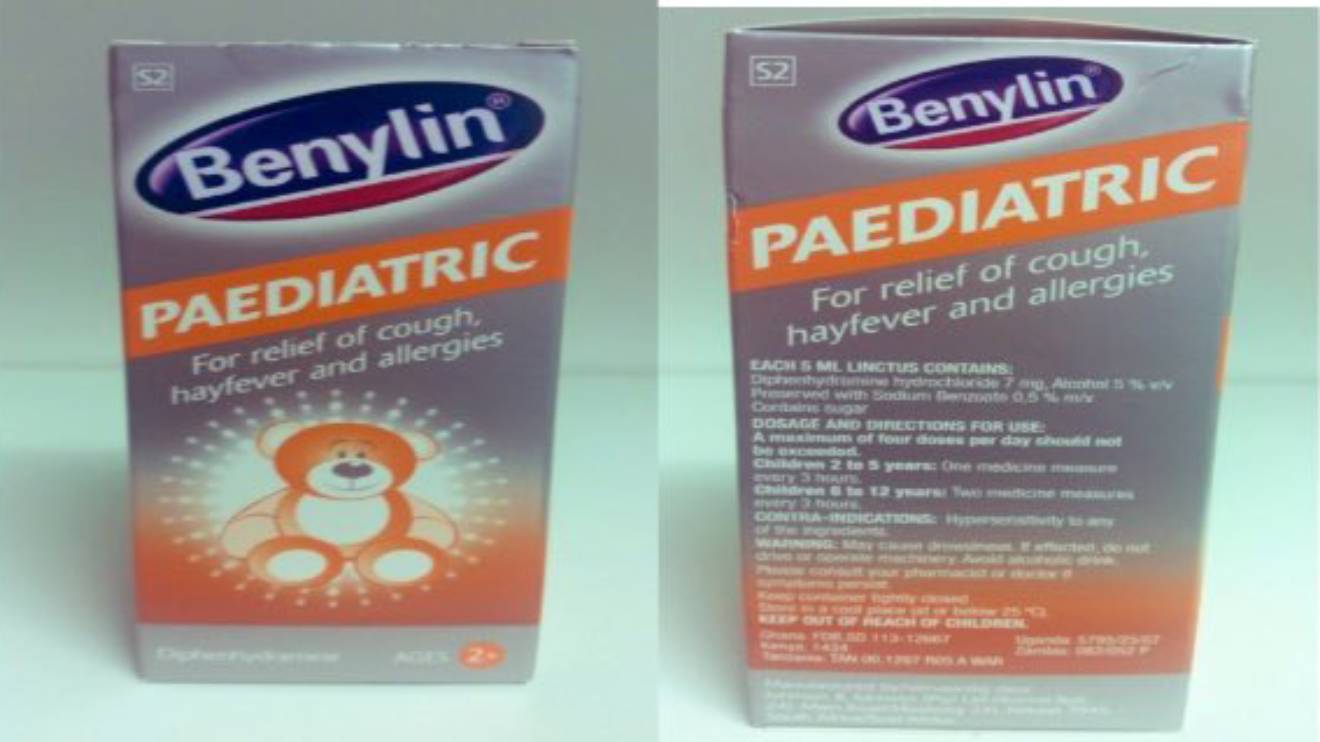
The Pharmacy and Poisons Board (PPB) of Kenya issued a public alert today regarding a specific batch of Benylin pediatric cough syrup.
The alert comes after the National Agency for Food and Drug Administration and Control (NAFDAC) of Nigeria initiated a recall of Benylin pediatric 100mls cough syrup, Batch No. 329304, manufactured by Johnson & Johnson (Pty) of South Africa.
"The Pharmacy and Poisons Board (PPB) wishes to inform the public that it has received information regarding a medical product alert and recall of Benylin pediatric 100mls cough syrup Batch No 329304, by the National Agency for Food and Drug Administration and Control (NAFDAC) of Nigeria," the board said in a statement.
"This cough syrup manufactured by Johnson & Johnson (Pty), South Africa, is being recalled due to quality concerns arising from an unacceptably high level of diethylene glycol detected through laboratory analysis conducted by NAFDAC."
The recall was prompted by the discovery of unacceptably high levels of diethylene glycol, a toxic contaminant, in the affected batch.
Read More
Consumption of diethylene glycol can lead to serious health problems, including abdominal pain, vomiting, diarrhoea, difficulty urinating, headaches, altered mental state, and potentially fatal acute kidney injury.
"Following receipt of this information the PPB has immediately commenced. investigations.," stated the Board.
"The PPB also initiated rapid response, which includes sampling of batches of Benylin pediatric 100mls syrup that are within shelf life for the purpose of screening of the levels of Ethylene glycol and diethylene glycol."
Important Actions for Public and Healthcare Providers:
Stoppage of Distribution and Use: The PPB urges all pharmacies, healthcare facilities, and the public to cease the distribution, sale, dispensing, and use of Benylin pediatric cough syrup, Batch No. 329304, immediately.
Product Return: The public is advised to return any affected bottles to the nearest healthcare facility. Healthcare facilities are instructed to return their stock to their respective suppliers.
Only Batch No. 329304 Affected: It's crucial to note that only Batch No. 329304 is affected by this recall. Other batches of Benylin pediatric cough syrup remain unaffected.
Reporting Adverse Reactions: Anyone experiencing adverse reactions in children following the use of this product is advised to seek immediate medical attention from a qualified healthcare professional.
The PPB assures the public of their commitment to safeguarding the quality, safety, and efficacy of medications available in Kenya.
They encourage continued vigilance and urge the reporting of any suspected poor-quality medicines or adverse drug reactions to the nearest healthcare facility or directly to the PPB.




-1720036750.jpg)





